As a paediatrican, I have always been passionate about helping prevent and control the spread of infectious diseases such as typhoid. I have been very lucky to recently get involved with the TyVAC project and have the opportunity to conduct research for my PhD on Salmonella Typhi (S. Typhi), the bacteria that causes typhoid fever.
While much of the work for the TyVAC studies in Bangladesh, Malawi, and Nepal takes place in a clinical or field setting, there is a lot of laboratory work going on behind the scenes. My colleagues at the Wellcome Trust Sanger Institute are studying DNA from typhoid strains found during the recent outbreak of extensively drug-resistant (XDR) typhoid in Pakistan. We are working to understand how the bacteria are adapting and causing disease in different places. With the rise of these XDR strains, vaccines are becoming more and more important in the fight against typhoid.
What makes typhoid so hard to study is that S. Typhi only causes disease in humans. For many bacteria, we can learn a considerable amount about what happens when they infect other animals, such as mice. Unfortunately, this is not possible for S. Typhi. We have to be creative and come up with other ways to find out what is going on when typhoid enters the body.
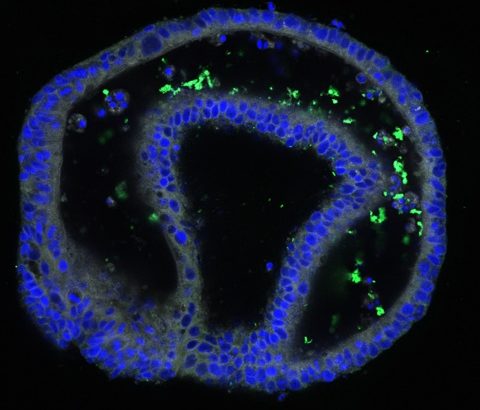
In my laboratory, we are using an exciting new technology called intestinal ‘organoids’, sometimes known as ‘mini-guts’, which are miniature models of the lining of the human intestine. Organoids form spherical structures, a maximum of two millimetres in diameter – somewhere between the size of a human hair and a flea – and contain all the different types of cells you would see in the human intestine. Organoids have cells that make mucus and cells that produce chemicals to attack invading bacteria. We use a tiny needle to inject S. Typhi into the middle of the organoids; powerful microscopes allow us to see how the bacteria attach to, and make their way through, the intestinal lining and into the body. This process replicates what happens when a person is infected with typhoid. Organoids complement and will hopefully enhance, our knowledge of how S. Typhi behaves in the human gut.
This injection technique allows us to study the genes the cells use to recognise and respond to infection; it can also be used to study other bacteria and viruses. It’s amazing to think that parts of our intestinal ecosystem can be re-created in these tiny collections of cells, enabling scientists to investigate how infections occur and even test new drugs. We are hopeful this information will help to identify new targets for vaccines and/or a test to diagnose and fight typhoid.
Laboratory work with organoids is extremely time intensive, but I am very excited to learn more about how the body fights this rapidly-adapting bacteria and to figure out how to add new weapons to our arsenal to take on typhoid. The more we learn and understand these processes, the better equipped we are to develop the necessary tools to investigate the disease and implement sound policy decisions to prevent and control typhoid.